
Is recognized as Mexico’s leading stem cell bank, holding the first federal license for stem cell banking in the country. It operates five national branches and ensures unparalleled quality standards. Dr. Jorge Mario Tagle, the Medical Director of CRC and President of the Mexican Council for Therapeutic and Research Stem Cells, oversees rigorous quality controls that guarantee patient safety and treatment efficacy.




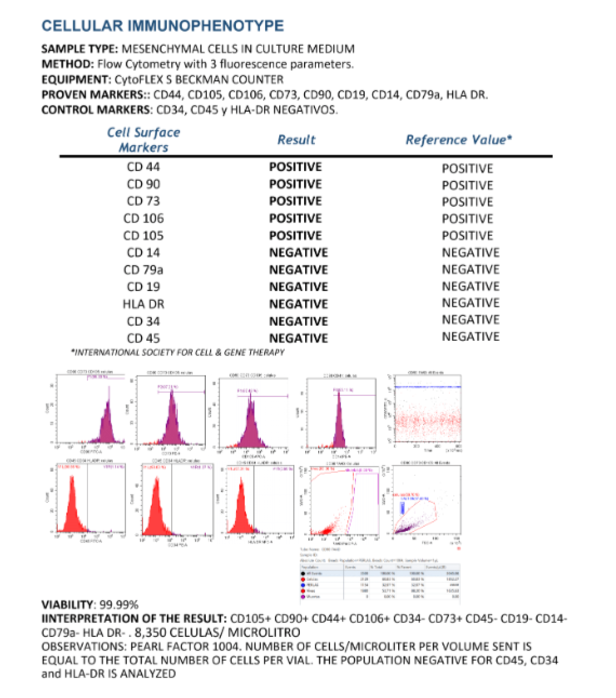
CRC’s collaboration with ITC guarantees that all stem cell treatments use cells from Mexico’s largest and most advanced bank. Having the same ownershp, CRC and ITC together provide the perfect patient treatment platform.
can learn more about ITC’s world-class facilities and processes here
Trustindex verifies that the original source of the review is Google. From the start to end process of being treated at CRC was tremendous customer service and as professional as you can ask for. If you are in search of stem cells regeneration look no further. Gideon is the MVP and if asked he will steer you to their favorite taco spot to eat!Trustindex verifies that the original source of the review is Google. Had a great experience here. Haven't felt any pain in my knee since the procedure months ago. Easy access and return across thd boarder. Highly recommended.Trustindex verifies that the original source of the review is Google. I’ve had a degenerating knee for many decades after a big ski accident and a number of operations. Skiing was becoming increasingly painful with a recurrent Baker’s Cyst. My last round of steroid jabs failed to make any difference so I went for the stem cell treatment in November last year. Just had a great ski season with a real, noticeable improvement in the inflammation and pain levels. Highly recommend the treatment and the company.Trustindex verifies that the original source of the review is Google. I had excellent care with Dr Portillo. I am waiting on the stem cells effectiveness since it will take a couple months to work for my problem. Good place to go.Trustindex verifies that the original source of the review is Google. This is place is TOP NOTCH! I came in on 12/1/23 after I was in the ER over the Thanksgiving weekend b/c of an issue with a bulging L5/S1. I had to be wheel-chaired in b/c I couldn't walk or even stand up straight and had lie in bed all day before I got my stem cell therapy. Gideon and the doctor talked to me about all the details and I got 25 million stem cells in my back and 25 million in a knee after a meniscus tear from August 2023 and some PRP. I felt IMMEDIATE pain relief and could return to normal movement. Now one month later I feel no pain and have returned to working out like I did previously. In the ER the doctor told me that it would take 12 weeks before I would feel this level of relief, but with CRC it happened 3x quicker. The cost was 25% of what I was quoted from other stem cell clinics and the building/facility was top notch. I will be recommending this place to anybody and everybody and plan to return again in early 2024 to have stem cells treat other injuries in my body.Trustindex verifies that the original source of the review is Google. was skeptical, as my injury was very severe and old. i shattered my ankle 30 years ago and had severe rheumatoid arthritis. my ankle is absolutely pain free. i’m not sure how it works, but i have benefited tremendously from this treatment.Trustindex verifies that the original source of the review is Google. The family is top notch & amazing. The service is wonderful & I would highly recommend to anyone!!Trustindex verifies that the original source of the review is Google. Very friendly...they explained everything..not crowded..I would recommended.Trustindex verifies that the original source of the review is Google. Getting treatment from CRC has been a game changer for me. I will forever be a patient. thanks Gideon and staff for making my visit quick, easy and so beneficial. Glenn Cadrez 11 year NFL linebacker.Trustindex verifies that the original source of the review is Google. I had a consultation with Dr. Robinson at CRC last week. My concern is that I have limited mobility in my shoulder from an injury from 2016. He suggested stem cell therapy and acupotomy sessions. I booked an appointment to move forward with the suggested treatment and I’m looking forward to seeing the results!
Outcomes will vary between individuals. No claims are being made with regenerative therapies.
The FDA considers stem cell therapy experimental.
Contact information
© 2024 Cellular Regeneration Clinic – All rights reserved.